
sodium alginate VS other hydrocolloids
What is sodium alginate?
Sodium alginate is one of important hydrocolloids which is extracted from brown seaweed like liminaria japonica, Lessonia Nigrescens (commonly named as LN seaweed), LessoniaTrabeculata (Flavicans) (commonly named as LF seaweed). It is a polymer formed by connecting β-D-mannuronic acid and α-L-guluronic acid by (1→4) bonds, and has good stability, solubility, viscosity and safety. Sodium alginate has been widely used in the food industry and the medical field, such as as a thickener, stabilizer, binder and drug delivery material. In addition, it is biodegradable and biocompatible, and can form gels in the presence of divalent ions. These characteristics make it play an important role in many fields.
The aqueous solution of sodium alginate has a high viscosity and has been used as a thickener, stabilizer, emulsifier in food. Sodium alginate is a non-toxic food and was included in the United States Pharmacopoeia as early as 1938. Sodium alginate contains a large amount of -COO-, which can exhibit polyanionic behavior in aqueous solution and has a certain degree of adhesion. It can be used as a drug carrier for treating mucosal tissue. Under acidic conditions, -COO- is converted into -COOH, the degree of ionization is reduced, the hydrophilicity of sodium alginate is reduced, and the molecular chain shrinks. When the pH value increases, the -COOH group continues to dissociate, the hydrophilicity of sodium alginate increases, and the molecular chain stretches. Therefore, sodium alginate has obvious pH sensitivity. Sodium alginate can quickly form a gel under extremely mild conditions. When cations such as Ca2+ and Sr2+ are present, the Na+ on the G unit undergoes an ion exchange reaction with the divalent cation, and the G unit accumulates to form a cross-linked network structure, thereby forming a hydrogel. The conditions for sodium alginate to form a gel are mild, which can avoid the inactivation of active substances such as sensitive drugs, proteins, cells and enzymes. Due to these excellent properties, sodium alginate has been widely used in the food industry and pharmaceutical field.
sodium alginate manufacturing process
The manufacturing process flow of sodium alginate is as follows: dry or wet seaweed (algae) is crushed, washed with water to remove impurities, extracted with strong alkaline water, and clarified to obtain a crude alginate solution, which is precipitated with calcium chloride to obtain calcium alginate, which is decolorized and deodorized and then treated with acid to remove soluble impurities to obtain alginate precipitate, which is reacted with sodium carbonate to obtain sodium alginate granular, and then dried, crushed, and sieved to obtain sodium alginate powder.
Physical and chemical properties
Sodium alginate is a white or light yellow powder, almost odorless and tasteless. Sodium alginate is soluble in water, but insoluble in organic solvents such as ethanol, ether, and chloroform. It dissolves in water to form a viscous liquid, and the pH value of a 1% aqueous solution is 6-8. When the pH is 6-9, the viscosity is stable, and the viscosity decreases when heated to above 80°C. Sodium alginate is non-toxic, and LD50>5000mg/kg. The effect of chelating agents on the properties of sodium alginate solutions Chelating agents can complex divalent ions in the system, making sodium alginate stable in the system.
1. Structure
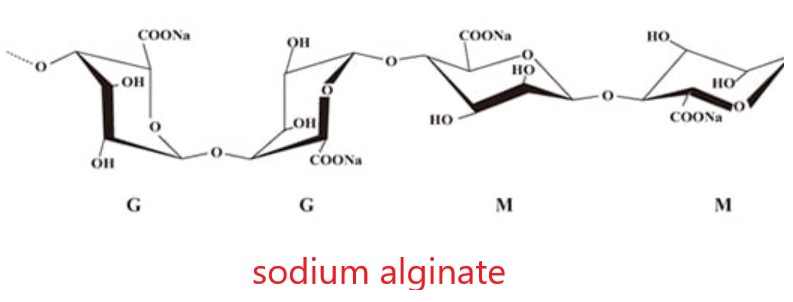
The molecular formula of sodium alginate is C6H7NaO6, which is mainly composed of the sodium salt of alginate, a copolymer composed of β-D-mannuronic acid (M unit) and α-L-guluronic acid (G unit) connected by β-1,4-glycosidic bonds and composed of GM, MM and GG fragments in different proportions.
2. Molecular weight
The molecular weight of sodium alginate is usually dispersed, like polysaccharides. Therefore, the molecular weight of sodium alginate usually represents the average of all molecules in the group. The most common ways to express molecular weight are number average molecular weight (Mn) and weight average molecular weight (Mw).
In a polydisperse molecular population, Mw>Mn is usually the coefficient of Mw/Mn. The coefficient of Mw/Mn is the dispersion index, and the index of commercial sodium alginate is typically in the range of 1.5 to 2.5. The most commonly used method to determine the molecular weight is calculated based on intrinsic viscosity and light scattering measurements.
3. Molecular formula
The molecular formula is C6H9NaO7, and the molecular structure is shown in Figure 1:
4. pH value
Sodium alginate is slightly soluble in water and insoluble in most organic solvents. It dissolves in alkaline solutions, making the solution viscous. Sodium alginate powder becomes wet when it meets water, and the hydration of the particles makes its surface viscous. The particles then quickly stick together to form clumps, which slowly completely hydrate and dissolve. Sodium alginate is more difficult to dissolve in water if the water contains other compounds that compete with alginate for hydration. Sugar, starch or protein in water will reduce the hydration rate of sodium alginate, and it is necessary to extend the mixing time. Salts of monovalent cations (such as NaCl) will also have a similar effect when the concentration is higher than 0.5%. The pH value of sodium alginate in a 1% distilled water solution is about 7.2.
5. Stability
Sodium alginate is hygroscopic, and the amount of water it contains at equilibrium depends on the relative humidity. Dry sodium alginate is quite stable when stored in a well-sealed container at 25°C or below. Sodium alginate solution is stable at pH 5-9. The degree of polymerization (DP) and molecular weight are directly related to the viscosity of sodium alginate solution. The decrease in viscosity during storage can be used to estimate the degree of depolymerization of sodium alginate. Sodium alginate with a high degree of polymerization is less stable than sodium alginate with a low degree of polymerization. It is reported that sodium alginate can be hydrolyzed by protons, and the hydrolysis depends on time, pH and temperature. Propylene glycol alginate solution is stable at room temperature and pH 3-4; when the pH is less than 2 or greater than 6, the viscosity will decrease rapidly even at room temperature.
6. Advantage
As a thickening agent for beverages and dairy products, sodium alginate has unique advantages in thickening: the good fluidity of sodium alginate makes the drink taste smooth after addition; and it can prevent the viscosity drop during the sterilization process of the product. When using sodium alginate as a thickener, products with larger molecular weights should be used as much as possible, and Ca should be added in an appropriate amount. It can greatly increase the viscosity of sodium alginate.
Sodium alginate is a high-grade stabilizer for ice cream and other cold drinks. It can give ice cream and other cold drinks a smooth appearance and smooth taste. Because calcium alginate can form a stable thermo-irreversible gel, it will not become rough (ice crystal growth) during transportation and storage, and ice cream will not deform due to temperature fluctuations. At the same time, this ice cream has no peculiar smell when eaten. It not only increases the expansion rate but also the melting point, significantly improving the quality and efficiency of the product. The product has a smooth, delicate texture and good taste. The addition amount is low, generally 1-3%, and the foreign addition amount is 5-10%.
Sodium alginate is used as a stabilizer for dairy products and beverages. Stabilized frozen milk has a good taste, no stickiness and stiffness, and is sticky and hysteretic when stirred.
7. sodium alginate applications
The role of sodium alginate in food mainly includes thickening, stabilization, improving taste and texture, and improving the nutritional value and freshness of food.
Thickening and stabilizing effect: Sodium alginate can form a stable gel network structure in food, has good moisturizing properties and taste improvement effects, and also has excellent water absorption and water retention, which can maintain the freshness and taste of food.
Improving taste and texture: In the food industry, sodium alginate is used as a thickener, stabilizer and gelling agent, which can make the taste of food more delicate, and at the same time improve the stability and preservation of food. In addition, sodium alginate can also be used to make low-fat foods and sugar-free foods because it has good film-forming and water-retaining properties.
Improving nutritional value and freshness: The coating preservative made of sodium alginate has gas selective permeability, which can prevent the food metabolism process, reduce the intensity of cell respiration and the consumption of nutrients, reduce the formation of reactive oxygen, reduce membrane lipid peroxidation, and protect food from foreign microorganisms, thereby achieving the purpose of food preservation.
Application in specific foods: When making seasonings, sodium alginate is mainly added to thicken and improve the texture; when making candied fruit and frosting, it mainly plays a stabilizing and thickening role; when making gel and pudding, it mainly acts as a curing agent; when making hard candy, it mainly acts as a stabilizer and thickener; when making fruit processed products and juices, sodium alginate can be added as a molding aid; when making noodles, macaroni, and vermicelli, it can play a role in hydration, enhancing tissue strength, cooking resistance, and refreshing; in the production of bread, pastries, and convenience foods, it plays a role in volume expansion, hydration, and tissue improvement.
In summary, sodium alginate, as a natural polysaccharide, has multiple functions and uses in the food industry, which can significantly improve the texture and shelf life of food, while improving the nutritional value and market competitiveness of food.

(2) Sodium alginate application in pharmaceutical preparations
Sodium alginate was included in the United States Pharmacopoeia as early as 1938. Alginic acid was included in the British Pharmacopoeia in 1963. Alginic acid is insoluble in water, but it swells when placed in water. Therefore, sodium alginate is traditionally used as a binder for tablets, while alginic acid is used as a disintegrant for immediate-release tablets. However, the effect of sodium alginate on tablet properties depends on the amount put into the prescription, and in some cases, sodium alginate can promote tablet disintegration. Sodium alginate can be added during the granulation process instead of adding it in the form of powder after granulation, which makes the production process simpler. Compared with the use of starch, the mechanical strength of the prepared tablets is greater.
Sodium alginate is also used in the production of suspensions, gels, and concentrated emulsions based on fats and oils. Sodium alginate is used in some liquid drugs to enhance viscosity and improve the suspension of solids. Propylene glycol alginate can improve the stability of emulsions.
(3) Sodium alginate application in the printing and textile industry
Sodium alginate is used as a reactive dye paste in the printing and dyeing industry, which is superior to grain starch and other slurries. The printed textiles have bright patterns, clear lines, high color yield, uniform color, good permeability and plasticity. Alginate is the best slurry in the modern printing and dyeing industry. It is now widely used in the printing of various fabrics such as cotton, wool, silk, nylon, etc., and is particularly suitable for preparing discharge printing paste.
The Chinese textile sector mixes alginate with starch or replaces starch to prepare warp yarn slurry, which can not only save a lot of food, but also make the warp yarn fiber non-pilling, friction-resistant, and less broken, thereby improving weaving efficiency. Alginate is effective for both cotton and synthetic fibers.

(4) Sodium alginate application in the pharmaceutical industry
PS type gastrointestinal double contrast barium sulfate preparation made of alginate sulfate dispersant has the characteristics of low viscosity, fine particle size, good wall adhesion and stable performance.
PSS is a kind of brown algae polysaccharide sodium ester developed with alginate as raw material, which has the effects of anticoagulation, lowering blood lipids and reducing blood viscosity.
Using alginate instead of rubber and gypsum as dental impression material is not only cheap and easy to operate, but also more accurate in tooth shape.
Alginate can also be used to make various dosage forms of hemostatic agents, including hemostatic sponges, hemostatic gauze, hemostatic films, scald gauze, spray hemostatic agents, etc.
Sodium alginate comparing with other hydrocolloids
Hydrocolloids refer to those that can be dissolved in water and hydrated under certain conditions to form a viscous and slippery solution or colloid. According to the different raw materials, it can be divided into animal colloids, plant colloids, seaweed colloids, microbial colloids, chemically modified colloids, etc. Common edible hydrocolloids include gelatin, carrageenan, xanthan gum, guar gum, gum arabic, agar, sodium alginate, locust bean gum and konjac gum. Edible hydrocolloids has a wide range of uses and can be applied to cold foods, beverages, dairy products, condiments, cakes, starch, candy, brewing, food preservation and refrigeration and other food industries. It can also be used in cosmetics, coatings, photosensitive resins, fertilizers, casting, tobacco and pharmaceutical industries. The following is a comparison of the gel properties of several hydrophilic colloids such as xanthan gum, sodium alginate, konjac gum, guar gum, CMC, modified starch, carrageenan, linseed gum, curdlan gum, gellan gum etc.
1. Xanthan gum
Xanthan gum is a monospore polysaccharide produced by the fermentation of Pseudocanthomonas. It is made of cabbage black rot wild rapeseed Xanthomonas with carbohydrates as the main raw material. After aerobic fermentation bioengineering technology, the 1,6-glycosidic bond is cut off, the side chain is opened, and then the 1,4-bond is used to synthesize a straight chain. It is an acidic extracellular heteropolysaccharide. Due to its special macromolecular structure and colloidal properties, it has multiple functions and can be used as an emulsifier, stabilizer, gel thickener, wetting agent, film forming agent, etc., and is widely used in various fields of the national economy. Xanthan gum can be quickly dissolved in cold water, but it has extremely strong hydrophilicity. Therefore, if it is not stirred sufficiently, the outer layer absorbs water and expands into micelles, which will prevent water from entering the inner layer. Therefore, xanthan gum dry powder or mixed with dry powder auxiliary materials such as salt and sugar, slowly add the stirring water to make a solution for use. The xanthan gum aqueous solution has high viscosity under static or low shearing action. Under high shearing action, the viscosity drops sharply, but the molecular structure remains unchanged. When the shearing force is eliminated, the original viscosity is immediately restored. Therefore, the xanthan gum solution has pseudoplasticity. The relationship between shearing force and viscosity is completely plastic. The pseudoplasticity of xanthan gum is very prominent, and this pseudoplasticity is extremely effective for stabilizing suspensions and emulsions. During the experiment, it was found that when xanthan gum was dissolved in cold water stirred with a glass rod, if it was added too quickly, the xanthan gum powder would not have time to fully diffuse and clump, and then it would be difficult to dissolve. When it was slowly added to the cold water stirred by a high-speed rotor, it was fully diffused, the clumps were not serious, and the solution after dissolution had high viscosity, slightly yellowed, and poor transparency.
Weigh 198g of 65°C hot water, stir with a high-speed rotor, add 2g of thickener, and observe the solubility of the thickener in hot water. (The same applies below)

2. Sodium alginate
Sodium alginate, also known as algin, and alginate, is a natural polysaccharide carbohydrate extracted from kelp. It is widely used in food, medicine, textiles, printing and dyeing, papermaking, daily chemical products, etc., as a thickener, emulsifier, stabilizer, adhesive, sizing agent, etc. Sodium alginate has strong hydrophilicity and can be dissolved in both cold and warm water to form a very viscous and uniform solution. The true solution formed has softness, uniformity and other excellent properties that are difficult to obtain with other analogs. It has a strong protective colloid effect and strong emulsification power for oils and fats. Experiments have found that sodium alginate is not easy to disperse in cold water. Although it is easy to clump in the upper layer of water, it is easy to dissolve. After dissolution, the solution has high viscosity and high transparency. Compounded sodium alginate is easier to clump than sodium alginate.

As can be seen from the figure, the dispersibility of sodium alginate in hot water is better than that in cold water, and it dissolves faster in hot water to form a uniform and transparent solution.
3. Konjac Gum
Konjac Gum is a hydrogel polysaccharide glucomannan (KGM) extracted from the tubers of various konjac plants. It is a high molecular weight, non-ionic KGM. The particles of konjac flour swell when exposed to water, then break and release the polymer of KGM. It is not only widely used as a food additive in the food industry, but also plays an important role in agriculture, medicine, and other industries. Experiments have found that konjac gum has good dispersibility and dissolves quickly under appropriate stirring and addition speeds, and forms a slightly powdery translucent solution after dissolution.

As can be seen from the figure, konjac gum has good dispersibility and solubility in hot water, but its transparency is not good, and konjac gum has a strong fishy smell when dissolved in hot water.
4. Guar gum
Guar gum is a non-ionic galactomannan obtained by cleaning, drying, crushing, adding water, and then pressurizing and hydrolyzing the endosperm of guar seeds, precipitating with 20% ethanol, centrifuging, drying, and crushing. Commercial gum is generally a white to light yellowish brown free-flowing powder, almost odorless, and has no other odor. It generally contains 75%~85% polysaccharides, 5%~6% protein, 2%~3% fiber and 1% ash. Guar gum can form a high-viscosity solution when dissolved in water, so it can be widely used in food, industry and medicine. Experiments have found that guar gum has good dispersibility and dissolves in water to form a slightly yellowish translucent solution.

5. Sodium Carboxymethyl Cellulose (CMC)
Sodium Carboxymethyl Cellulose (CMC) is usually an anionic polymer compound obtained by reacting natural cellulose with caustic soda and monochloroacetic acid. It is the sodium salt of cellulose carboxymethyl ether and has a molecular weight of 6400 (±1000). CMC is a modified natural cellulose. The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) have officially called it "modified cellulose". CMC is white or milky white fibrous powder or granules, with a density of 0.5-0.7 g/cm3, almost odorless and tasteless, hygroscopic, easy to disperse in water into a transparent colloidal solution, insoluble in organic solvents such as ethanol, and has the functions of bonding, thickening, strengthening, emulsifying, water retention, and suspension. It was found in the experiment that CMC has poor dispersibility when dissolved in cold water and is easy to clump. Therefore, CMC should be evenly spread and stirred continuously when used. CMC is easy to produce bubbles after being dissolved in high-speed stirring cold water, and a uniform transparent solution is formed after standing for a period of time.

6. Modified starch
On the basis of the inherent properties of natural starch, in order to improve the performance of starch and expand its application range, physical, chemical or enzymatic treatment is used to introduce new functional groups on starch molecules or change the size of starch molecules and the properties of starch particles, thereby changing the natural properties of starch (such as gelatinization temperature, thermal viscosity and its stability, freeze-thaw stability, gel strength, film-forming property, transparency, etc.) to make it more suitable for certain application requirements. This kind of starch that has undergone secondary processing and changed its properties is collectively referred to as modified starch. At present, there are more than 2,000 varieties and specifications of modified starch. The classification of modified starch is generally based on the treatment method, including oxidized starch, acid-modified starch, starch ester, starch ether, cross-linked starch, cationic starch, grafted starch, cyclodextrin, white dextrin, pregelatinized starch (pregelatinized starch), dialdehyde starch, etc. Among them, there are more than 200 kinds of modified starch produced with corn starch, while there are only more than ten varieties of modified starch produced with corn starch as raw material in inland China. As one of the important raw materials in industry, modified starch can be widely used in papermaking, food, textile, construction, medicine and other industries. Modified starch is mainly used as thickener, gelling agent, adhesive, emulsifier and stabilizer in the food industry.

7. Carrageenan
Carrageenan is a hydrophilic colloid extracted from certain red algae seaweeds. Its chemical structure is composed of calcium, potassium, sodium, and ammonium salts of polysaccharide sulfates composed of galactose and dehydrated galactose. Due to the different binding forms of sulfates, it can be divided into K type (Kappa), I type(lota), and L type (Lambda). It is widely used in the manufacture of jelly, ice cream, cakes, soft candy, canned food, meat products, eight-treasure porridge, white fungus and bird's nest, soup foods, cold foods, etc. Carrageenan is insoluble in cold water, but can swell into a gel block. It is insoluble in organic solvents and easily soluble in hot water to form a translucent colloidal solution (the dissolution rate increases in hot water above 70°C), and can form a thermally irreversible gel. It has a synergistic effect with colloids such as locust bean gum, konjac gum, and xanthan gum, which can improve the elasticity and water retention of the gel. The experiment found that carrageenan is insoluble in cold water and contains relatively many impurities; refined carrageenan is slightly soluble in cold water and appears as fine flocs.


